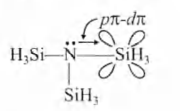Q.
Account for the following. Write the answers in four or five sentences only.
(i) The experimentally determined $N-F$ bond lengths in $NF_3$ is greater than the sum of the single bond covalent radii of $N$ and $F$.
(ii) $Mg_3N_2$ when reacted with water gives of $NH_3$ but $HCl$ is not obtained from $MgCl_2$ on reaction with water at room temperature.
(iii) $(SiH_3)_3 N $ is a weaker base than $(CH_3 )_3 N.$
IIT JEEIIT JEE 1995The p-Block Elements - Part2
Solution: