- Tardigrade
- Question
- Physics
- A thermodynamic system is taken from an initial state i with internal energy U i =100 J to the final state f along two different paths iaf and ibf, as schematically shown in the figure. The work done by the system along the paths a f,ib and bf are W af =200 J , W ib =50 J and W bf =100 J respectively. The heat supplied to the system along the path iaf, ib and bf are Q textiaf, Q textib and Q text bf respectively. If the internal energy of the system in the state b is U b =200 J and Q text iaf =500 J, the ratio Q textbf / Q textib is
Q.
A thermodynamic system is taken from an initial state with internal energy to the final state along two different paths iaf and , as schematically shown in the figure. The work done by the system along the paths and are and respectively. The heat supplied to the system along the path iaf, and are , and respectively. If the internal energy of the system in the state is and , the ratio is
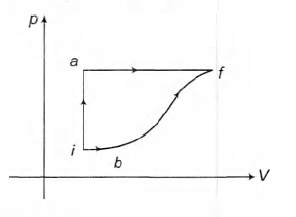
Answer: 2
Solution:
Process iaf
Process
W(in Joule)
( in Joule )s
Q(in Joule)
ia
0
af
200
Net
300
200
500
Joule Process ibf
Process
W(in Joule)
( in Joule )
Q(in Joule)
ib
100
50
150
bf
200
100
300
Net
300
150
450
| Process | W(in Joule) | ( in Joule )s | Q(in Joule) |
|---|---|---|---|
| ia | 0 | ||
| af | 200 | ||
| Net | 300 | 200 | 500 |
| Process | W(in Joule) | ( in Joule ) | Q(in Joule) |
|---|---|---|---|
| ib | 100 | 50 | 150 |
| bf | 200 | 100 | 300 |
| Net | 300 | 150 | 450 |