- Tardigrade
- Question
- Physics
- A sample of 2 kg monoatomic helium (assumed ideal) is taken through the process ABC and another sample of 2 kg of the same gas is taken through the process ADC (see fig). Given molecular mass of helium = 4. (a) What is the temperature of helium in each of the states A, B,C and D? (b) Is there any way of telling afterwards which sample of helium went through the process ABC and which went through the process ADC? Write Yes or No. (c) How much is the heat involved in the process ABC and ADC?
Q.
A sample of 2 kg monoatomic helium (assumed ideal) is taken
through the process ABC and another sample of 2 kg of the
same gas is taken through the process ADC (see fig). Given
molecular mass of helium = 4.
(a) What is the temperature of helium in each of the states
A, B,C and D?
(b) Is there any way of telling afterwards which sample of
helium went through the process ABC and which went
through the process ADC? Write Yes or No.
(c) How much is the heat involved in the process ABC and
ADC?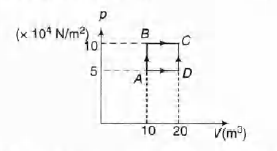
Solution: