- Tardigrade
- Question
- Physics
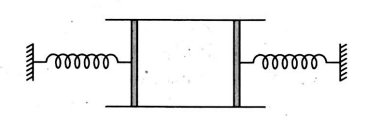
- A cylinder of cross-sectional area A has two pistons of negligible mass separated by distances l and loaded with spring of negligible mass. An ideal gas at temperature T1 is in the cylinder where the springs are relaxed. When the gas is heated by some means its temperature becomes T2 and the springs get compressed by (l/2) each. If P0 is the atmospheric pressure and spring constant is k=(2 P0 A/l), then find the ratio of T2 and T1 .
Q.
A cylinder of cross-sectional area has two pistons of negligible mass separated by distances and loaded with spring of negligible mass. An ideal gas at temperature is in the cylinder where the springs are relaxed. When the gas is heated by some means its temperature becomes and the springs get compressed by each. If is the atmospheric pressure and spring constant is , then find the ratio of and
Answer: 4
Solution: