- Tardigrade
- Question
- Chemistry
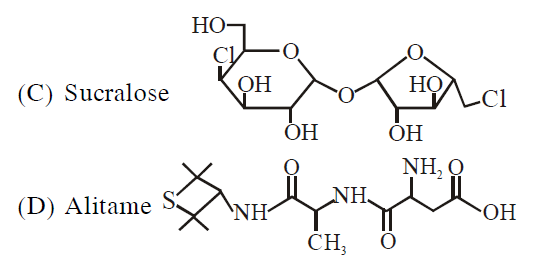
- A chemist has 4 samples of artificial sweetener A, B, C and D. To identify these samples, he performed certain experiments and noted the following observations: (i) A and D both form blue-violet colour with ninhydrin. (ii) Lassaigne extract of C gives positive AgNO3 test and negative Fe4[Fe(CN)6]3 test. (iii) Lassaigne extract of B and D gives positive sodium nitroprusside test. Based on these observations which option is correct ?
Q.
A chemist has samples of artificial sweetener and . To identify these samples, he performed certain experiments and noted the following observations :
(i) and both form blue-violet colour with ninhydrin.
(ii) Lassaigne extract of gives positive test and negative test.
(iii) Lassaigne extract of and gives positive sodium nitroprusside test.
Based on these observations which option is correct ?
Solution:
(i) Blue voilet color with Ninhydrine amino acid derivative. So it cannot be saccharide or sucralose.
(ii) Lassaigne extract give +ve test with So Cl is present, -ve test with means N is absent. So it can't be Aspartame or Saccharine or Alitame, so C is sucralose.
(iii) Lassaigne solution of B and D given +ve sodium nitroprusside test, so it is having S, so it is Saccharine and Alitame.