Q.
A chemist has $4$ samples of artificial sweetener $A, B, C$ and $D$. To identify these samples, he performed certain experiments and noted the following observations :
(i) $A$ and $D$ both form blue-violet colour with ninhydrin.
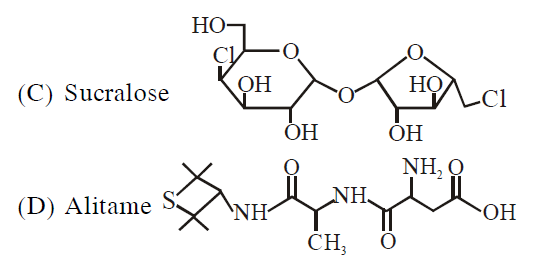
(ii) Lassaigne extract of $C$ gives positive $AgNO_3$ test and negative $Fe_4[Fe(CN)_6]_3$ test.
(iii) Lassaigne extract of $B$ and $D$ gives positive sodium nitroprusside test.
Based on these observations which option is correct ?
Solution:
(i) Blue voilet color with Ninhydrine $\to$ amino acid derivative. So it cannot be saccharide or sucralose.
(ii) Lassaigne extract give +ve test with $AgNO_{3}.$ So Cl is present, -ve test with $Fe_{4}\left[Fe\left(CN\right)_{6}\right]_{3}$ means N is absent. So it can't be Aspartame or Saccharine or Alitame, so C is sucralose.
(iii) Lassaigne solution of B and D given +ve sodium nitroprusside test, so it is having S, so it is Saccharine and Alitame.