- Tardigrade
- Question
- Physics
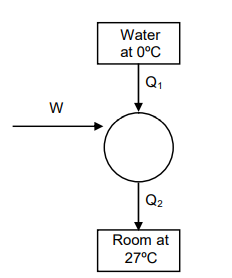
- A Carnot freezer takes heat from water at 0°C inside it and rejects it to the room at a temperature of 27°C. The latent heat of ice is 336 × 103 J kg-1. If 5 kg of water at 0°C is converted into ice at 0°C by the freezer, then the energy consumed by the freezer is close to :
Q. A Carnot freezer takes heat from water at inside it and rejects it to the room at a temperature of . The latent heat of ice is . If of water at is converted into ice at by the freezer, then the energy consumed by the freezer is close to :
Solution: