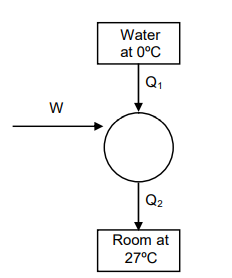
Q. A Carnot freezer takes heat from water at $0^{\circ}C$ inside it and rejects it to the room at a temperature of $27^{\circ}C$. The latent heat of ice is $336 \times 10^3 J \, kg^{-1}$. If $5\, kg$ of water at $0^{\circ}C$ is converted into ice at $0^{\circ}C$ by the freezer, then the energy consumed by the freezer is close to :
Solution: