- Tardigrade
- Question
- Physics
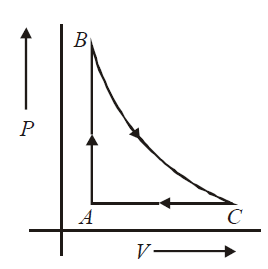
- 0.2 moles of an ideal gas is taken round the cycle A B C as shown in the figure. The path B arrow C is an adiabatic process, A arrow B is an isochoric process and C arrow A is an isobaric process. The temerature at A and B are TA=300 K and TB=500 K and pressure at A is 1 atm and volume at A is 4.9 L. The volume at C is (Given: γ=(Cp/CV)=(5/3), R = 8.205 Ã 10-2 L atm mol-1 K-1, ((3/2))2/5 =0.81
Q.
moles of an ideal gas is taken round the cycle as shown in the figure. The path is an adiabatic process, is an isochoric process and is an isobaric process. The temerature at and are and and pressure at is 1 atm and volume at is . The volume at is
(Given :
Solution: