Q. Which of the following have been arranged in decreasing order of oxidation number of sulphur?
NTA AbhyasNTA Abhyas 2020The p-Block Elements - Part2
Solution:
Compound Oxidation state $H_{2}SO_{5}$ $+6$ $H_{2}SO_{3}$ $+4$ $SCl_{2}$ $+2$ $H_{2}S$ $-2$
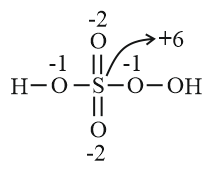
| Compound | Oxidation state |
|---|---|
| $H_{2}SO_{5}$ | $+6$ |
| $H_{2}SO_{3}$ | $+4$ |
| $SCl_{2}$ | $+2$ |
| $H_{2}S$ | $-2$ |