Q.
The volume versus temperature graph of $1$ mole of an ideal gas is given below
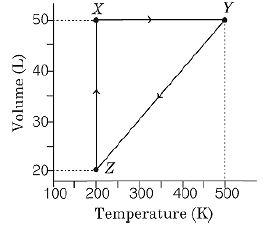
The pressure of the gas (in atm) at $X$ , $Y$ and $Z,$ respectively, are
KVPYKVPY 2015Solutions
Solution: