Q.
The reaction $A \longrightarrow C$ proceeds through two paths I and II as shown below :
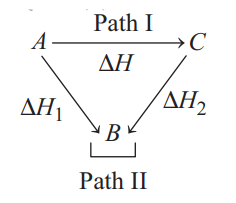
The correct relationship between $\Delta H, \Delta H_{1}$ and $\Delta H_{2}$ will be
Thermodynamics
Solution: