Q.
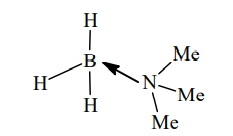
The geometry around boron in the product 'B' formed from the following reaction is
$ BF _3+ NaH \xrightarrow{450 K } A + NaF$
$ A + NMe _3 \rightarrow B$
Solution:
$BF_3 + NaH \xrightarrow{450\,K} \underset{\text{(diborane)}}{B_2H_6} + NaF$
$B_2H_6 + NMe_3 \rightarrow \underset{\text{symmetrical cleavage}}{2[BH_3 \leftarrow NMe_3]}$