Q. The correct structure of $Fe ( CO )_{5}$ is
Bihar CECEBihar CECE 2012Coordination Compounds
Solution:
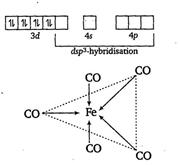
In $Fe ( CO)_{5}$, the Fe atom is in $dsp ^{3}$ hybridised state.
Therefore, the shape of molecule is trigonal bipyramidal.
The hybridisation is as
${ }_{26} Fe =1 s^{2},\, 2 s^{2} 2 p^{6},\, 3 s^{2} 3 p^{6} 3 d^{6},\, 4 s^{2} 4 p^{0}$
In $Fe ( CO )_{5}$ the $'Fe'$ atom is