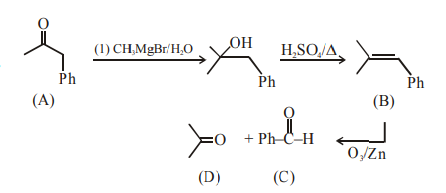
Q.
The compound A in the following reaction is :
$A \xrightarrow[(ii)Conc.H_2SO_4/\Delta]{(i) CH_3MgBr/H_2O}$
$B \xrightarrow[(ii)Zn/H_2O]{(i)O_3} C + D$

$D \xrightarrow[\Delta]{Ba(OH)_2}H_3C-\overset{\underset{|}{C}H_3}{C} = CH - \overset{\underset{||}{O}}{C}-CH_3$
Solution: