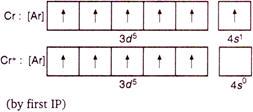
Q. The atomic numbers of vanadium $ (V) $ , chromium $ (Cr) $ , manganese $ (Mn) $ and iron $ (Fe) $ are respectively $ 23,\,\,24,\,\,25 $ and $ 26 $ . Which one of these may be expected to have the highest second ionisation enthalpy?
Rajasthan PMTRajasthan PMT 2008
Solution:
This is stable $ EC $ , hence formation of $ C{{r}^{2+}} $ by second $ IP $ requires maximum enthalpy.