Q.
Suppose $0.5$ mole of an ideal gas undergoes an isothermal expansion as energy is added to it as heat $Q$. Graph shows the final volume $V_{f}$ versus $Q$. The temperature of the gas
is $\left(\right.$ use $\ln 9=2$ and $\left. R=\frac{25}{3} J / mol - K \right)$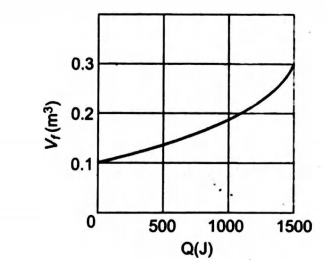
Thermodynamics
Solution: