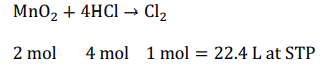Q.
In the following reaction,
$MnO _2+4 HCl \rightarrow MnCl _2+2 H _2 O + Cl _2$
$2$ moles $MnO _2$ react with $ 4$ moles of $HCl$ to form $11.2\, L\, Cl _2$ at STP. Thus, per cent yield of $Cl _2$ is
Some Basic Concepts of Chemistry
Solution: