Q.
In a cylindrical container of sufficiently large height, two easily moving pistons enclose certain amount of same ideal gas in two chambers as shown in the figure-2.43.
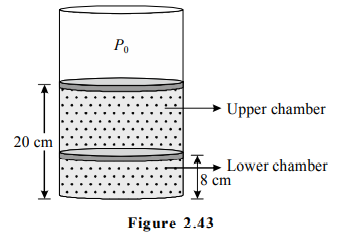
The upper piston is at a height $20\, cm$ from the bottom and lower piston is at a height $8\, cm$ from the bottom. The mass of each piston is m kg and cross sectional area of each piston is $A\, m^2$ where $\frac{m g}{A}=P_{0}$ and $P_{0}$ is the atmospheric pressure $=1 \times 10^{5} N / m ^{2}$.
The cylindrical container and pistons are made of conducting material. Initially the temperature of gas is $27^{\circ} C$ and whole system is in equilibrium. Now if the upper piston is slowly lifted by $16\, cm$ and held in that position with the help of some external force. As a result, the lower piston rises slowly by $l\, cm$.
In a cylindrical container of sufficiently large height, two easily moving pistons enclose certain amount of same ideal gas in two chambers as shown in the figure-2.43.

The upper piston is at a height $20\, cm$ from the bottom and lower piston is at a height $8\, cm$ from the bottom. The mass of each piston is m kg and cross sectional area of each piston is $A\, m^2$ where $\frac{m g}{A}=P_{0}$ and $P_{0}$ is the atmospheric pressure $=1 \times 10^{5} N / m ^{2}$.
The cylindrical container and pistons are made of conducting material. Initially the temperature of gas is $27^{\circ} C$ and whole system is in equilibrium. Now if the upper piston is slowly lifted by $16\, cm$ and held in that position with the help of some external force. As a result, the lower piston rises slowly by $l\, cm$.
The value of $l$ is :
Kinetic Theory
Solution: