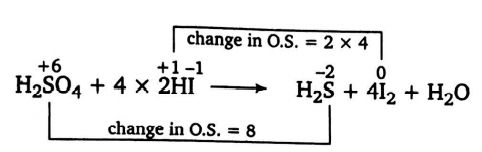Q.
In a balanced equation,
$H_{2}SO_{4}+xHI \rightarrow H_{2}S +yI_{2}+zH_{2}O$
the value of $ x,\,y$ and $z$ are respectively
Punjab PMETPunjab PMET 2011Redox Reactions
Solution: