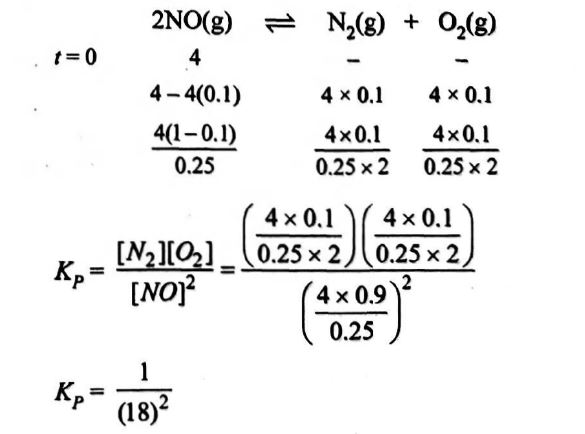
Q. In a $0.25$ litre tube dissociation of $4$ moles of $NO$ takes place. If its degree of dissociation is $10\%$. The value of $K$ for reaction $2NO \rightleftharpoons N_{2} + O_{2}$ is:
Equilibrium
Solution:
Correct answer is (a) $\frac {1}{(18)^{2}}$