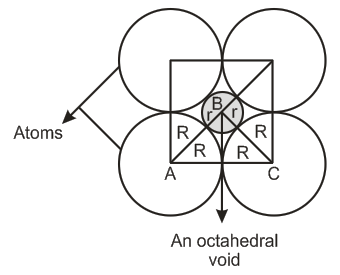
Q. If the radius of octahedral voids and radius of the atoms in close-packing are r and R, respectively. Derive the relation between r and R. What is the ratio of $100\times \frac{r}{R}.$ (Give answer to the nearest integer.)
NTA AbhyasNTA Abhyas 2022
Solution: