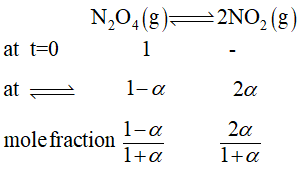Q. For the reaction $N_{2}O_{4}\rightleftharpoons2NO_{2 \left(g\right)}$ , the degree of dissociation of $N_{2}O_{4}$ is $0.2$ at $1atm.$ Then the $K_{p}$ of $2NO_{2}\rightleftharpoons N_{2}O_{4}$ is
NTA AbhyasNTA Abhyas 2020Equilibrium
Solution: