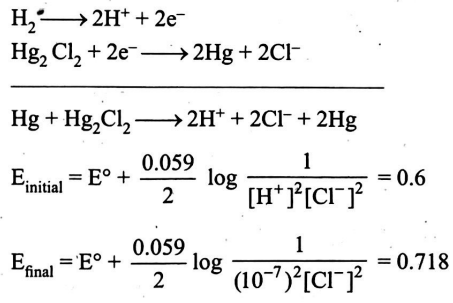Q. For the cell $Pt \left| H _{2}( g )\right|$ solution $X \| KCl$ (saturated) $\left| Hg _{2} Cl _{2}\right| Hg \mid Pt$, the observed $EMF$ at $25^{\circ} C$ was $600\, mV$. When solution $X$ was replaced by a standard phosphate buffer with $pH =7.00$, the $EMF$ was $718\, mV$. Find the $pH$ of solution $X$.
Electrochemistry
Solution: