Q.
For Daniell cell,
$Zn\left(\right.s\left.\right)+Cu^{2 +}\left(\right.aq\left.\right)\rightleftharpoonsCu\left(\right.s\left.\right)+Zn^{2 +}\left(\right.aq\left.\right)$
The reaction quotient Q is given by
$Q=\frac{\left[\right. Z n^{2 +} \left]\right.}{\left[\right. C u^{2 +} \left]\right.}$
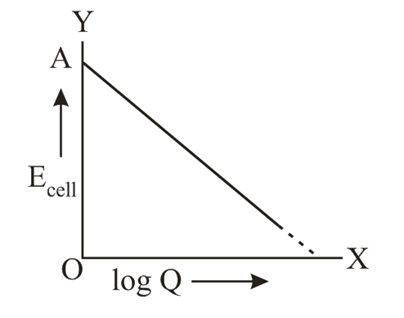
OA = 1.10 volt
Where $E_{c e l l}=1.1591$ volt, then
NTA AbhyasNTA Abhyas 2022
Solution: