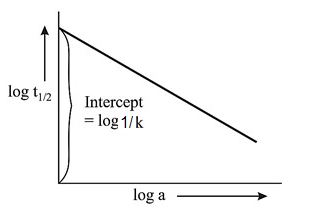
Q. For a second order reaction, $2A \rightarrow $ Products, a plot of $\log t_{1 / 2}$ vs $\log a$ (where, $a$ is initial concentration) will give an intercept equal to
NTA AbhyasNTA Abhyas 2022
Solution:
Solution: