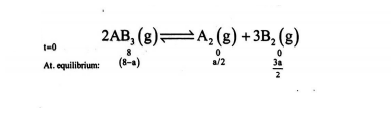Q. Eight mole of a gas $AB _{3}$ attain equilibrium in a closed container of , volume $1 dm ^{3}$ as, $2 AB _{3} \rightleftharpoons A _{2}( g )+3 B _{2}( g ) $ If at equilibrium $2 mole$ of $A_{2}$ are present then, equilibrium constant is:
Equilibrium
Solution: