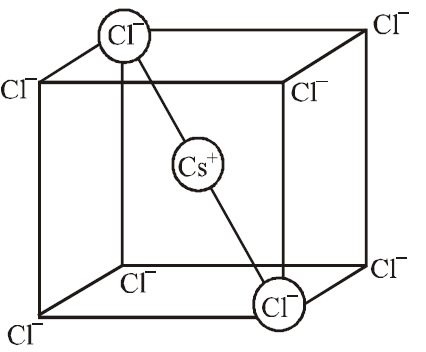
Q. CsCl crystallises in body centred cubic lattice. If ‘a’ is its edge length then which of the following expressions is correct?
Solution:
Relation between radius of cation, anion and edge length of the cube
$2 r _{ Cs^+}+2 r _{ Cl^- }=\sqrt{3} a$
$r _{ Cs ^+}+ r _{ Cl ^-}=\frac{\sqrt{3} a}{2}$