Q.
A fixed mass of ideal gas undergoes changes of pressure and volume starting at L, as shown in figure
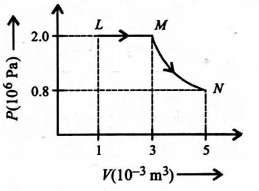
Which graph shows how temperature (measured in Kelvin) changes with volume?
Thermodynamics
Solution: