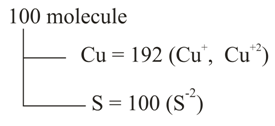Q. A certain sample of cuprous sulphide is found to have the composition $Cu_{1 . 92}S_{1 . 00}$ because of incorporation of $Cu^{2 +}$ and $Cu^{+}$ ions in the crystal then ratio of $Cu^{2 +}$ and $Cu^{+}$ ions is:
NTA AbhyasNTA Abhyas 2020
Solution: