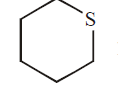
Q.
$0.102\, g$ of an organic compound $X$ was oxidized with fuming nitric acid. The resulting solution, after reaction with an excess of aqueous $BaCl _{2}$, produced $0.233 \,g$ of $BaSO _{4}$ as a precipitate. Compound $X$ is likely to be :
[Given: Atomic wt. of $Ba =137]$
KVPYKVPY 2020
Solution: