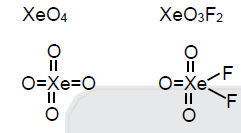Q.
Which of the following number of lone pair at central atom zero
XeO3, XeO2F2, XeO4, XeO3F2, Ba2XeF4
Solution:
both does not have lone pair of electrons
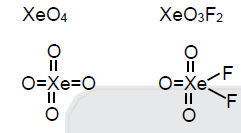
Solution: