- Tardigrade
- Question
- Chemistry
- Which of the following is a positive overlap that leads to bonding? (I) <img class=img-fluid question-image alt=Question src=https://cdn.tardigrade.in/q/nta/c-iyxkpzpwi9bq.png /> (II) <img class=img-fluid question-image alt=Question src=https://cdn.tardigrade.in/q/nta/c-iyxkpzpwmsvu.png /> (III) <img class=img-fluid question-image alt=Question src=https://cdn.tardigrade.in/q/nta/c-iyxkpzpw28q3.png /> (IV) <img class=img-fluid question-image alt=Question src=https://cdn.tardigrade.in/q/nta/c-iyxkpzpwpf7f.png />
Q.
Which of the following is a positive overlap that leads to bonding?
(I)
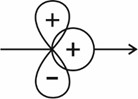
(II)
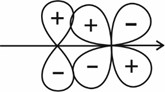
(III)
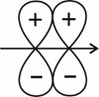
(IV)
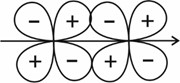
Solution: