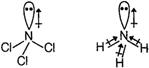
Q. Which of the following has maximum dipole moment?
Solution:
Electronegativity difference between N (3.0) and Cl (3.0) is zero and hence, N-Cl bonds are non-polar. As a result, the overall dipole moment of molecule and its direction is just the dipole moment of the lone pair of electrons.
On the other hand, N-Br, (3.0 - 2.8) N-l (3.0 - 2.5) and N-H (3.0 - 2.1) bonds are polar and hence, contribute towards the overall dipole moment of the respective molecules. Since, the EN difference is higher in case of N-H bonds, therefore, has the higher dipole moment.