- Tardigrade
- Question
- Physics
- The three processes in a thermodynamic cycle shown in the figure are: Process 1arrow 2 is isothermal; Process 2 arrow 3 is isochoric (volume remains constant); Process 3 arrow 1 is adiabatic <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/physics/349b3782779a40186b7ad5b583ef45a5-.jpeg /> The total work done by the ideal gas in this cycle is 10 J. The in ternal energy decreases by 20 J in the isochoric process. The work done by the gas in the adiabatic process is -20 J. The heat added to the system in the isothermal process is
Q.
The three processes in a thermodynamic cycle shown in the figure are :
Process is isothermal;
Process is isochoric (volume remains constant);
Process
is adiabatic
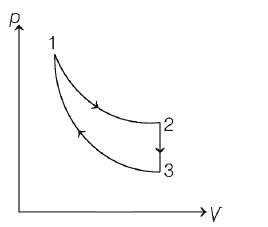
The total work done by the ideal gas in this cycle is The in ternal energy decreases by in the isochoric process. The work done by the gas in the adiabatic process is The heat added to the system in the isothermal process is
Solution:
