Q.
The three processes in a thermodynamic cycle shown in the figure are :
Process $1\rightarrow 2$ is isothermal;
Process $2 \rightarrow 3$ is isochoric (volume remains constant);
Process
$3 \rightarrow 1$ is adiabatic
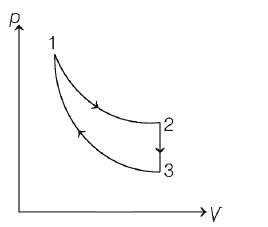
The total work done by the ideal gas in this cycle is $10\, J.$ The in ternal energy decreases by $20 \,J$ in the isochoric process. The work done by the gas in the adiabatic process is $-20\,J.$ The heat added to the system in the isothermal process is
KVPYKVPY 2013Thermodynamics
Solution:
