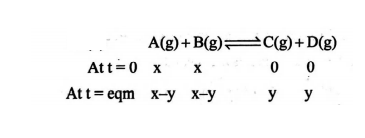- Tardigrade
- Question
- Chemistry
- The reaction, A ( g )+ B ( g ) leftharpoons C ( g )+ D ( g ) occurs in a single step. The rate constant of forward reaction is 2 × 10-3 mol -1 L sec -1 . When the reaction is started with equimolar amounts of A B, it is found that the concentration of A is twice that of C at equilibrium. The rate constant of backward reaction is:
Q. The reaction, occurs in a single step. The rate constant of forward reaction is When the reaction is started with equimolar amounts of , it is found that the concentration of is twice that of at equilibrium. The rate constant of backward reaction is:
Solution: