- Tardigrade
- Question
- Chemistry
- The direct conversion of A to B is difficult, hence it is carried out by the following shown path <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/chemistry/a6c283d5d0e416a455053c61218476f3-.png /> Given that Δ S(A arrow C)=50 eu Δ S(C arrow D)=30 eu Δ S(D arrow B)=-20 eu where, eu is entropy unit Then, Δ S(A arrow B) is
Q.
The direct conversion of to is difficult, hence it is carried out by the following shown path
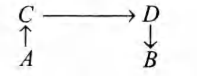
Given that eu
eu
eu
where, eu is entropy unit
Then, is
Solution: