Q.
The direct conversion of $A$ to $B$ is difficult, hence it is carried out by the following shown path
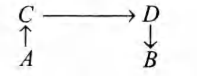
Given that $\Delta S_{(A \rightarrow C)}=50$ eu
$\Delta S_{(C \rightarrow D)}=30$ eu
$\Delta S_{(D \rightarrow B)}=-20$ eu
where, eu is entropy unit
Then, $\Delta S_{(A \rightarrow B)}$ is
IIT JEEIIT JEE 2006Thermodynamics
Solution: