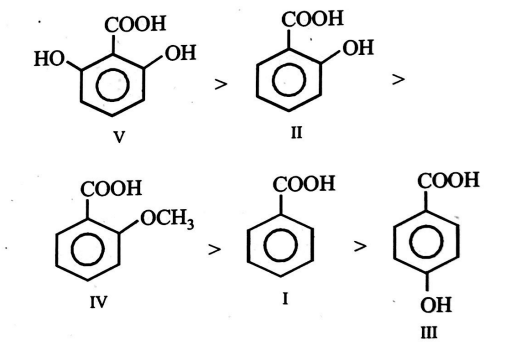
Q.
The correct order for the acidic character of the following carboxylic acids is
Solution:
is most stable because its anion is stabilized to a greater extent through - bonding with atom of present on both ortho-positions ; followed by II in which one group is present. Compound IV comes next to II because here group is present in ortho position which although is not capable of forming -bonding yet more acidic than (III) due to ortho effect. Compound III is less acidic than benzoic acid because of electron-releasing group in the para position. Thus