- Tardigrade
- Question
- Chemistry
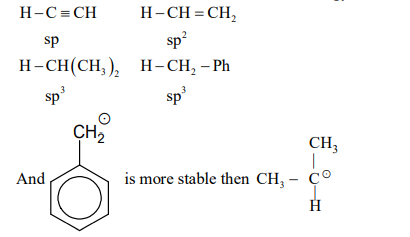
- The amount of energy required to break a bond is same as the amount of energy released when the same bond is formed. In gaseous state, the energy required for homolytic cleavage of a bond is called Bond Dissociation Energy (BDE) or Bond Strength. BDE is affected by s-character of the bond and the stability of the radicals formed. Shorter bonds are typically stronger bonds. BDEs for some bonds are given below: <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/chemistry/3d8c17e1040b9b6c5435e02bc7048f81-.png /> Correct match of the C-H bonds (shown in bold) in Column J with their BDE in Column K is Column J Molecule Column II K BDE(kal mol-1) P H - CH(CH3)2 i 132 Q H - CH2Ph ii 110 R H - CH = CH2 iii 95 S H - C ≡ CH iv 88
Q.
The amount of energy required to break a bond is same as the amount of energy released when the same bond is formed. In gaseous state, the energy required for homolytic cleavage of a bond is called Bond Dissociation Energy (BDE) or Bond Strength. BDE is affected by -character of the bond and the stability of the radicals formed. Shorter bonds are typically stronger bonds. BDEs for some bonds are given below:

Correct match of the bonds (shown in bold) in Column with their BDE in Column is
Column Molecule
Column II BDE(
P
i
132
Q
ii
110
R
iii
95
S
iv
88
| Column Molecule | Column II BDE( | ||
|---|---|---|---|
| P | i | 132 | |
| Q | ii | 110 | |
| R | iii | 95 | |
| S | iv | 88 | |
Solution: