Q.
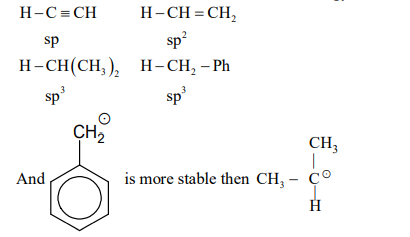
The amount of energy required to break a bond is same as the amount of energy released when the same bond is formed. In gaseous state, the energy required for homolytic cleavage of a bond is called Bond Dissociation Energy (BDE) or Bond Strength. BDE is affected by $s$-character of the bond and the stability of the radicals formed. Shorter bonds are typically stronger bonds. BDEs for some bonds are given below:

Correct match of the $C-H$ bonds (shown in bold) in Column $J$ with their BDE in Column $K$ is
Column $J$ Molecule
Column II $K$ BDE($kal \, mol^{-1})$
P
$H - CH(CH_3)_2$
i
132
Q
$H - CH_2Ph$
ii
110
R
$H - CH = CH_2$
iii
95
S
$H - C \equiv CH$
iv
88
| Column $J$ Molecule | Column II $K$ BDE($kal \, mol^{-1})$ | ||
|---|---|---|---|
| P | $H - CH(CH_3)_2$ | i | 132 |
| Q | $H - CH_2Ph$ | ii | 110 |
| R | $H - CH = CH_2$ | iii | 95 |
| S | $H - C \equiv CH$ | iv | 88 |
JEE AdvancedJEE Advanced 2021
Solution: