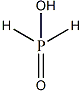
Q. Strong reducing behaviour of is due to
Solution:
Strong reducing behavior of is due to the presence of one - group and two bonds.
The reducing properties of oxyacids of phosphorous are due to the presence of bond. It has high tendency to release protons. Thus exhibits reducing nature.