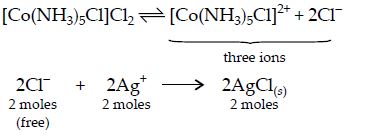Q. One mole of the complex compound , gives three moles of ions on dissolution in water. One mole of the same complex reacts with two moles of to yield two moles of . the structure of the complex is
Solution:
Correct answer is (a)
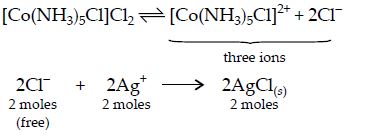
Solution: