- Tardigrade
- Question
- Chemistry
- One mole of an ideal gas is taken from a to b along two paths denoted by the solid and the dashed lines as shown in the graph below. If the work done along the solid line path is ws and that along the dotted line path is wd, the integer closest to the ratio wd/ws is <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/chemistry/c8ca0c44e77a25a77e8e21c17405d27d-.png />
Q.
One mole of an ideal gas is taken from to along two paths denoted by the solid and the dashed lines as shown in the graph below. If the work done along the solid line path is and that along the dotted line path is , the integer closest to the ratio is
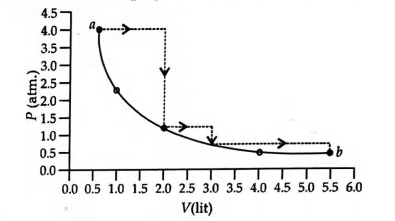
Solution: