Q.
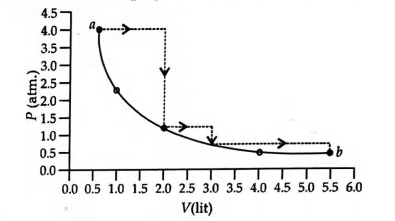
One mole of an ideal gas is taken from $a$ to $b$ along two paths denoted by the solid and the dashed lines as shown in the graph below. If the work done along the solid line path is $w_{s}$ and that along the dotted line path is $w_{d}$, the integer closest to the ratio $w_{d}/w_{s}$ is
Thermodynamics
Solution: