- Tardigrade
- Question
- Physics
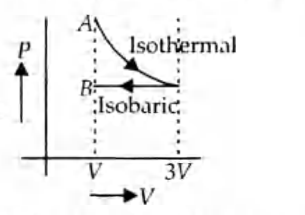
- One mole of an ideal gas goes from an initial state A to final state B via two processes: It first undergoes isothermal expansion from volume V to 3 V and then its volume is reduced from 3 V to V at constant pressure. The correct P-V diagram representing the two processes is:
Q. One mole of an ideal gas goes from an initial state to final state via two processes : It first undergoes isothermal expansion from volume to and then its volume is reduced from to at constant pressure. The correct diagram representing the two processes is:
Solution:
Correct answer is (d)