- Tardigrade
- Question
- Physics
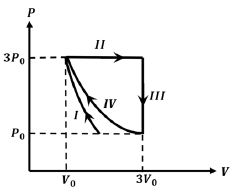
- One mole of a monatomic ideal gas undergoes four thermodynamic processes as shown schematically in the PV - diagram below. Among these four processes, one is isobaric, one is isochoric, one is isothermal and one is adiabatic. Match the processes mentioned in List-I with the corresponding statements in List-II. List-I List-II P. In process I 1. Work done by the gas is zero Q. In process II 2 Temperature of the gas remains unchanged R. In process III 3 No heat is exchanged between the gas and its surroundings S. In process IV 4 Work done by the gas is 6 P0 V0
Q.
One mole of a monatomic ideal gas undergoes four thermodynamic processes as shown schematically in the - diagram below. Among these four processes, one is isobaric, one is isochoric, one is isothermal and one is adiabatic. Match the processes mentioned in List-I with the corresponding statements in List-II.
List-I
List-II
P.
In process I
1.
Work done by the gas is zero
Q.
In process II
2
Temperature of the gas remains unchanged
R.
In process III
3
No heat is exchanged between the gas and its surroundings
S.
In process IV
4
Work done by the gas is
| List-I | List-II | ||
|---|---|---|---|
| P. | In process I | 1. | Work done by the gas is zero |
| Q. | In process II | 2 | Temperature of the gas remains unchanged |
| R. | In process III | 3 | No heat is exchanged between the gas and its surroundings |
| S. | In process IV | 4 | Work done by the gas is |
Solution: