- Tardigrade
- Question
- Chemistry
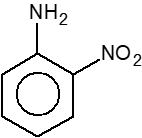
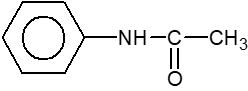
- How many compounds are less basic than aniline (.i.) <img class=img-fluid question-image alt=Question src=https://cdn.tardigrade.in/q/nta/c-i57zsbndrwsp.png /> (.ii.) <img class=img-fluid question-image alt=Question src=https://cdn.tardigrade.in/q/nta/c-i57zsbndlp59.png /> (.iii.) <img class=img-fluid question-image alt=Question src=https://cdn.tardigrade.in/q/nta/c-i57zsbndmscq.png /> (.iv.) NH3 (.v.) <img class=img-fluid question-image alt=Question src=https://cdn.tardigrade.in/q/nta/c-i57zsbndffol.png /> (.vi.) <img class=img-fluid question-image alt=Question src=https://cdn.tardigrade.in/q/nta/c-i57zsbndxmuy.png />
Q.
How many compounds are less basic than aniline




2518
266
NTA AbhyasNTA Abhyas 2020Organic Chemistry – Some Basic Principles and Techniques
Report Error
Answer: 4
Solution: