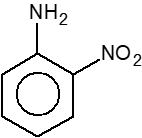
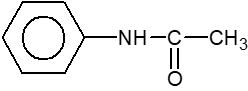
Q.
How many compounds are less basic than aniline
$\left(\right.i\left.\right)$

$\left(\right.ii\left.\right)$

$\left(\right.iii\left.\right)$

$\left(\right.iv\left.\right)$ $NH_{3}$
$\left(\right.v\left.\right)$

$\left(\right.vi\left.\right)$
NTA AbhyasNTA Abhyas 2020Organic Chemistry – Some Basic Principles and Techniques
Solution: